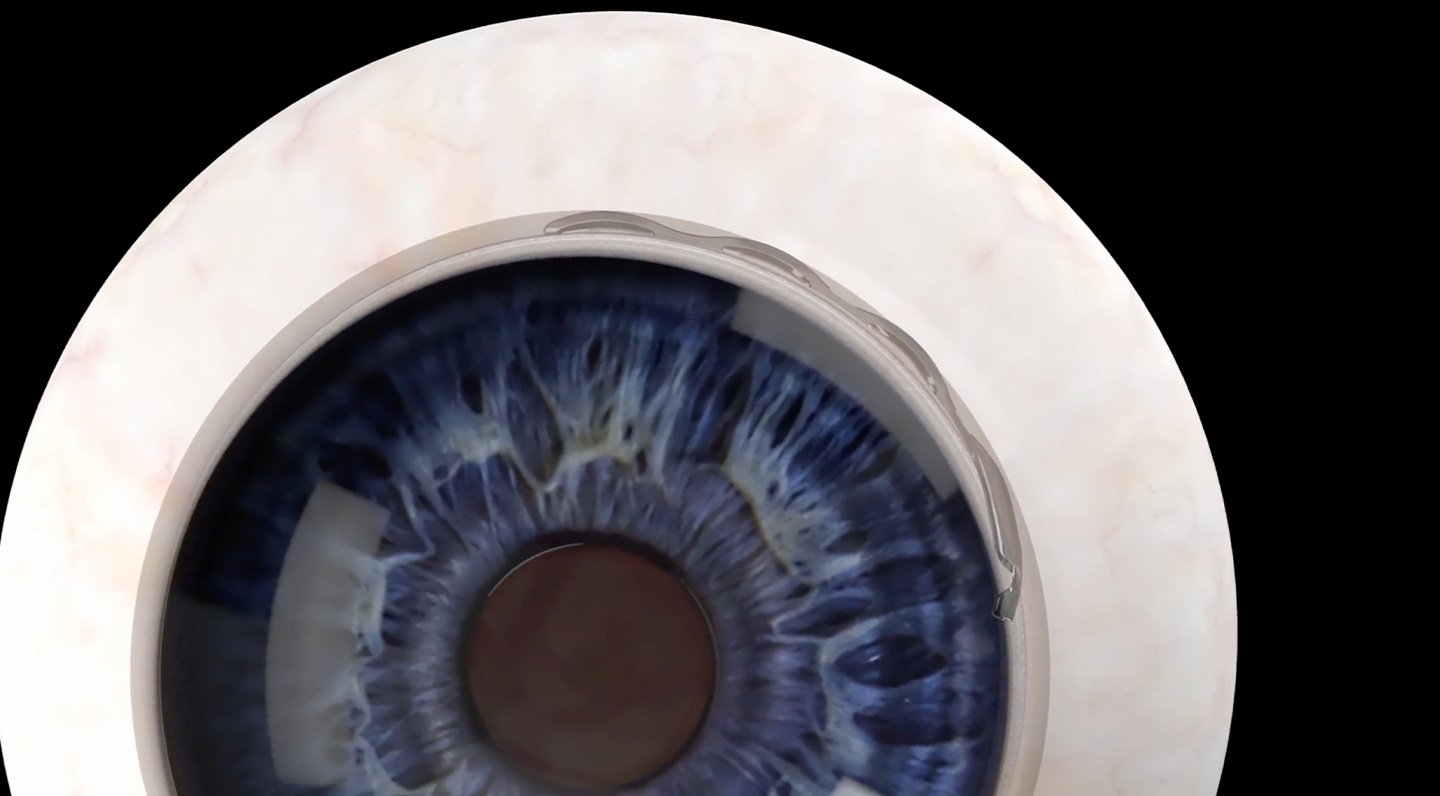
Chosen as an investigator for the Ivantis Hydrus FDA clinical trial.
Congratulations to Dr. Wandling, chosen as an investigator for the Ivantis Hydrus FDA clinical trial. Dr. Wandling’s selection makes Twin Cities Eye Consultants and North Metro Surgery Center one of only 15 sites across the United States active in this prospective, post-approval FDA study, designed to determine the ongoing safety of the device. It will soon be wrapping up its 24-month study results.
Dr. Wandling is a leading expert and one of the most experienced cataract and cataract/MIGS surgeons in the upper Midwest. TCEC is proud of his accomplishments, research, and contributions to the field of ocular surgery.
The Hydrus is a MIGS (Microinvasive Glaucoma Surgery) device implanted at the time of cataract surgery used to treat mild to moderate open-angle glaucoma.